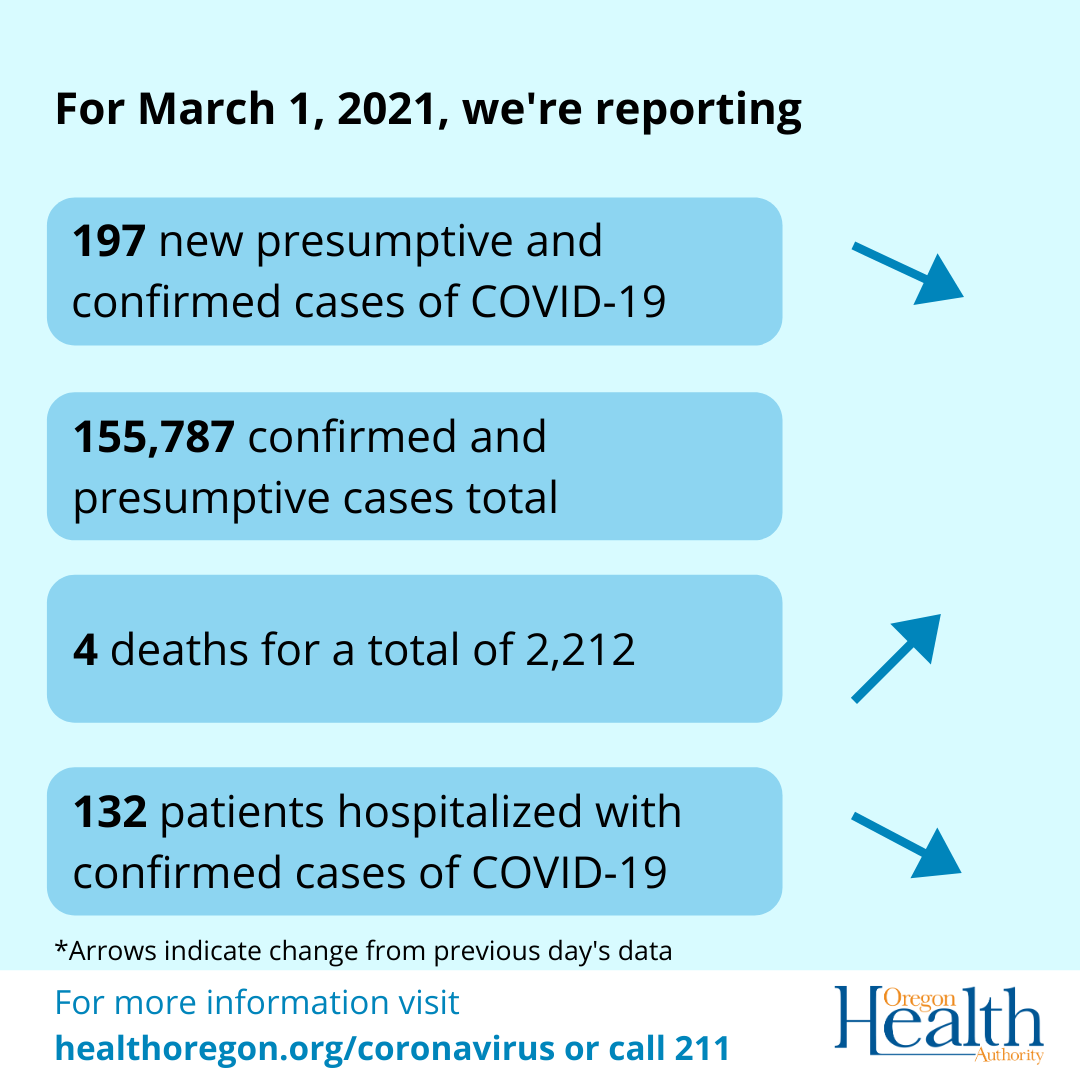
PORTLAND, Ore. — There are four new COVID-19 related deaths in Oregon, raising the state’s death toll to 2,212, the Oregon Health Authority reported today March 1, 2021.
Oregon Health Authority reported 197 new confirmed and presumptive cases of COVID-19, bringing the state total to 155,787.
The new confirmed and presumptive COVID-19 cases reported are in the following counties: Baker (3), Clackamas (19), Columbia (5), Coos (6), Deschutes (4), Douglas (11), Jackson (12), Jefferson (2), Josephine (3), Lane (33), Lincoln (1), Linn (2), Marion (20), Multnomah (16), Polk (3), Umatilla (1), Washington (54) and Yamhill (1).
Oregon to receive Johnson & Johnson vaccine
Johnson & Johnson’s single-dose vaccine has received an Emergency Use Authorization (EUA) from the federal government, making it the third COVID-19 vaccine available for use in the United States.
The Johnson & Johnson vaccine is the first single-dose vaccine against COVID-19. It can be stored in a refrigerator for months, making it easier to distribute without the need for ultra-cold storage.
OHA estimates Oregon will receive 34,000 doses of the Johnson & Johnson vaccine this week. OHA is working with Local Public Health Authorities, state retail pharmacy partners and hospital systems to administer the vaccine.
It is anticipated that less of the Johnson & Johnson vaccine will be available in the next few weeks following this week’s initial allocation. OHA is planning for strategic deployment of the vaccine to speed up vaccinations in Oregon.
“Having access to a third highly effective COVID-19 vaccine is a game changing development for Oregonians,’ said Paul Cieslak, M.D, medical director for communicable diseases and immunization, OHA Public Health Division. “We believe this vaccine is effective against the virus, and a one-dose regimen will allow us to vaccinate more Oregonians more quickly.”
The process for the Johnson & Johnson vaccine review and approval was the same as it was for the Moderna and Pfizer vaccines. The company submitted its application for EUA on Feb. 4.
In its review of Johnson & Johnson’s application, the FDA reported the vaccine was 66% effective for moderate to severe/critical COVID-19 in all groups across all regions studied starting at 28 days after vaccination. The observed efficacy in the United States was 72%. The clinical trial involved 43,783 participants in the United States, Latin America, Brazil and South Africa.
“The best thing is that this one-dose vaccine was 85% efficacious in preventing severe COVID-19,” Dr. Cieslak said.
Reported vaccine side effects include pain at the injection site, mild to moderate headache, fatigue and muscle aches.
Vaccinations in Oregon
Today, OHA reported that 13,794 new doses of COVID-19 vaccinations were added to the state immunization registry. Of this total, 6,169 doses were administered on Feb. 28 and 7,625 were administered on previous days but were entered into the vaccine registry on Feb. 28.
Cumulative daily totals can take several days to finalize because providers have 72 hours to report doses administered and technical challenges have caused many providers to lag in their reporting. OHA has been providing technical support to vaccination sites to improve the timeliness of their data entry into the state’s ALERT Immunization Information System (IIS).
Oregon has now administered a cumulative total of 986,816 first and second doses of COVID-19 vaccines. To date, 1,241,415 doses of vaccine have been delivered to sites across Oregon.
These data are preliminary and subject to change. OHA’s dashboards provide regularly updated vaccination data, and Oregon’s dashboard has been updated today.
COVID-19 hospitalizations
The number of hospitalized patients with COVID-19 across Oregon is 132, which is two fewer than yesterday. There are 27 COVID-19 patients in intensive care unit (ICU) beds, which is one more than yesterday.
The total number of patients in hospital beds may fluctuate between report times. The numbers do not reflect admissions per day, nor the length of hospital stay. Staffing limitations are not captured in this data and may further limit bed capacity.
More information about hospital capacity can be found here.
Test reporting change provides more detailed estimate of COVID-19 testing in Oregon
OHA continues to adapt how it reports COVID test results to provide a more detailed estimate of testing volume and percent positivity. The change will add test results reported via the Oregon COVID-19 Reporting Portal (OCRP) to the current Electronic Lab Reports (ELRs) totals.
OHA changed from the person-based test counts (i.e., number of people who test positive, negative, total people tested) to test-based counts on Dec. 3 by reporting the number of positive, negative and total COVID-19 electronic laboratory reports, representing the majority of COVID-19 test results reported statewide.
COVID-19 test results may also be reported by the secure, web-based confidential reporting system: Oregon COVID-19 Reporting Portal (OCRP). These reports were automatically routed to the appropriate local health department for public health action. Recent database improvements have made reporting these additional data possible.
These additional testing data will be published starting today in the Tableau dashboards and in risk level metrics for schools and counties. While there may be some changes to previously reported test positivity rates, case counts, and case rates have not changed.
OHA recommends that all Oregonians continue to follow the safe practices to prevent the spread of COVID-19. That includes wearing a mask or face covering, maintain physical distancing, minimize indoor social get-togethers, stay home if you feel sick, and frequently wash your hands.
Learn more about COVID-19 vaccinations
To learn more about the COVID-19 vaccine situation in Oregon, visit our webpage, which has a breakdown of distribution and other useful information.